A Story of One Scientist – Discovery Leading to Lithium-ion Batteries –
2018/12/19 Toshiba Clip Team
Smartphones, laptops, electric cars, all products essential for contemporary life, and all powered by batteries. Lithium-ion batteries to be precise. Compact and light, yet capable of supplying a powerful charge, the lithium-ion battery overturned the notion that rechargeable batteries must be large and heavy.
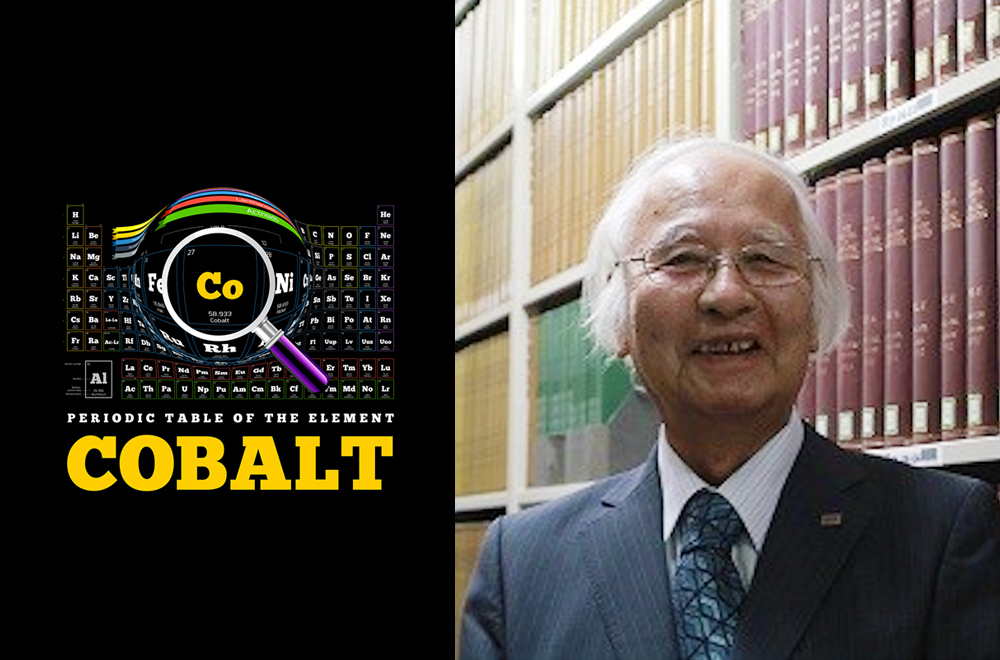
At 77, Koichi Mizushima is spry and active and still working, lending his years of knowledge and experience to Toshiba Research Consulting Corporation as a specially appointed Executive Fellow. In a life of many achievements, one of the high points was his participation in the project team that realized the lithium-ion battery, a game-changer that gave us today’s world of go-everywhere portable products. This is his story about how today’s indispensable power source was born.
What is a lithium-ion battery?
The essential characteristics of the lithium-ion battery are its large energy density and ability to supply a high voltage charge despite being light and compact. Those qualities, and rechargeability, make it ideal for use as a power supply for a wide range of products over a long period of time.
Switch on a device powered by a lithium-ion battery and the battery releases positively charged ions and negatively charged electrons that flow between electrodes, from a positive anode to a negative cathode, and from there into the circuit of the device as electricity. Critical to how well this works are the materials used in the electrodes.
In 1978, Mizushima, then a post-doc physics researcher at Tokyo University, was invited to Oxford University to join John B. Goodenough’s research team, which was investigating materials for battery anodes. It was a major project. The potential of lithium-ion batteries was well known, but the big question was what materials were best suited for the electrodes. The team’s research work focused on identifying and testing candidate materials. That proved to be more difficult than initially imagined.

Seeing Things from a Different Angle
In the late 1970s, the best lithium batteries had a lithium metal cathode and a sulfide anode. The superior performance of sulfides over other materials used in anodes intrigued Mizushima and his fellow researchers, to the point where they borrowed a furnace from an adjacent laboratory to synthesize sulfides for themselves. However, that didn’t turn out too well. One day, an experiment resulted in an explosion and a fire in the laboratory, a setback that prompted second thoughts. The team shifted its research focus to metal oxides, which were much safer to synthesize.
Having tested a range of oxides of metals such as iron and manganese, Mizushima made the discovery that lithium cobalt oxide was the best material. Rather than pursuing the orthodox approach of looking for materials that could absorb lithium ions, he turned the problem on its head and searched for materials from which lithium ions could be extracted. Cobalt turned out not only to fit the bill, but also to be capable of delivering voltages of up to 4V, nearly twice the power other batteries could generate.
It was a discovery that turbocharged lithium-ion battery research.

Breakthrough for a Safer Battery
Of course, one discovery is often the introduction to another problem. In this case, it was weak current flow. Any commercial lithium-ion battery would need a higher current density. Once again, it was a problem that proved difficult to crack, but once again the team persevered. In the end they found the solution is to shaving the electrode sheets to a thickness of around 100 microns. This breakthrough resulted in a lithium-ion battery that generated an electric current similar to that of conventional batteries, but with double the voltage and energy storage.
Functionally, the lithium-ion battery completely outperformed its rivals at the time. The anode not only improved performance by realizing high voltages, it did it much this more safely than other batteries. The new battery design also replaced the potentially hazardous lithium metal in the cathode, boosting safety without any loss of energy density.
The research gave the world the first lithium-ion battery free of lithium metal.
Lessons for younger scientists
Companies around the world have built on the foundations of the research results published by Mizushima and his fellow researchers to tweak and improve the lithium-ion battery. Indispensable as they have become the lithium-ion batteries continue to evolve, and have been refined to the point where they are even used as power supplies in space.
Limits have emerged to the use of lithium-ion batteries as high-capacity storage batteries, though. Further advances will be required to enable greater energy capacity and more rapid charging so that battery-powered electric vehicles can compete with gasoline-powered vehicles. While various next-generation candidates have emerged, such as the zinc-air battery, but so far none has demonstrated a decisive advantage over the lithium-ion battery. Attempts to claim superiority for alternative battery designs have tended to underscore the elegance of the lithium-ion battery solution.
“Looking back 30 years, it amazes me that someone hadn’t discovered this structurally simple anode material of lithium cobalt oxide earlier,” recalls Mizushima. “At the time, many oxides had already been tested as anode materials, and I wonder how many other researchers considered the same compound. I think it would likely have been only a matter of months before another researcher zeroed in on the material.”
As Mizushima recalls, many researchers were searching for anode materials. However, in line with earlier research, most were looking for materials that could absorb lithium ions, and might have put off searching for other types of materials. Adopting an unconventional perspective was the key to research yielding results in this case.
Behind Mizushima’s achievements, there may be hints for the continued progress of science. The challenge remains for scientists to aim higher for the next round of advances.
![]()
Related Links
*This section contains links to websites operated by companies and organizations other than Toshiba Corporation.
https://www.reuters.com/brandfeatures/road-to-a-new-day/powering-smart-cities-with-lithium-ion-batteries?utm_source=referral&utm_medium=toshibaclip&utm_campaign=prtoshibaclip&utm_term